Glycol for chillers is especially important to maintain the quality of chilled water supplied, avoid freezing fluid issues, prevent equipment deterioration, and ensure proper heat exchange performance.
Industrial glycol consists of ethylene glycol or propylene glycol, a corrosion inhibitor, and water. It is added to closed-loop water systems for protection against freezing, scaling, and pipe degradation.
Water and Glycol Concentration
Ethylene Glycol:
Ethylene glycol is an efficient antifreeze protector and generally less expensive than propylene glycol, but it is toxic. Therefore, installations must adhere to appropriate safety protocols during application or disposal.
Propylene Glycol:
This is a non-toxic alternative to ethylene glycol but is more costly. For installations that handle food or those with eco-friendly initiatives, this is the obvious choice.
Maintenance Aspects of Glycol in Chillers
- Check Glycol Pressure:
Verifying glycol pressure is a good way to assess pump functionality and ensure the chiller is delivering the normal glycol amount. - Inspect Glycol Levels in the Tank:
Low glycol levels can lead to significant problems across the entire chiller. Glycol levels can be checked using an external observation tube. - Measure Glycol Mixture:
Glycol mixtures are measured using a refractometer. Maintaining a mixture of 26.5 Brix (35% propylene glycol and 65% water) is essential for optimal performance. - Freezing Point Testing:
Test the freezing point of the mixture. Adjust or replace the mixture as needed. Typically, glycol levels range from 20% to 25%; however, consult the manufacturer’s recommendations for specific guidance. - Assess pH Levels:
The ideal pH should be between 8 and 10. If pH is too low, additional inhibitors may be required, or the system may need to be flushed and refilled. - Avoid Automotive Antifreeze:
Automotive antifreeze is unsuitable for chillers as it can cause flow rate issues and reduce heat transfer efficiency.
Glycol Concentration Chart
- Propylene Glycol vs Freezing Point:
| Percentage | Freezing Temp. (°C) | Freezing Temp. (°F) |
|---|---|---|
| 0% | 0°C | 32°F |
| 10% | -3°C | 26.6°F |
| 20% | -8°C | 17.6°F |
| 30% | -14°C | 6.8°F |
| 40% | -22°C | -7.6°F |
| 50% | -34°C | -29.2°F |
| 60% | -48°C | -54.4°F |
| 100% | -59°C | -74.2°F |
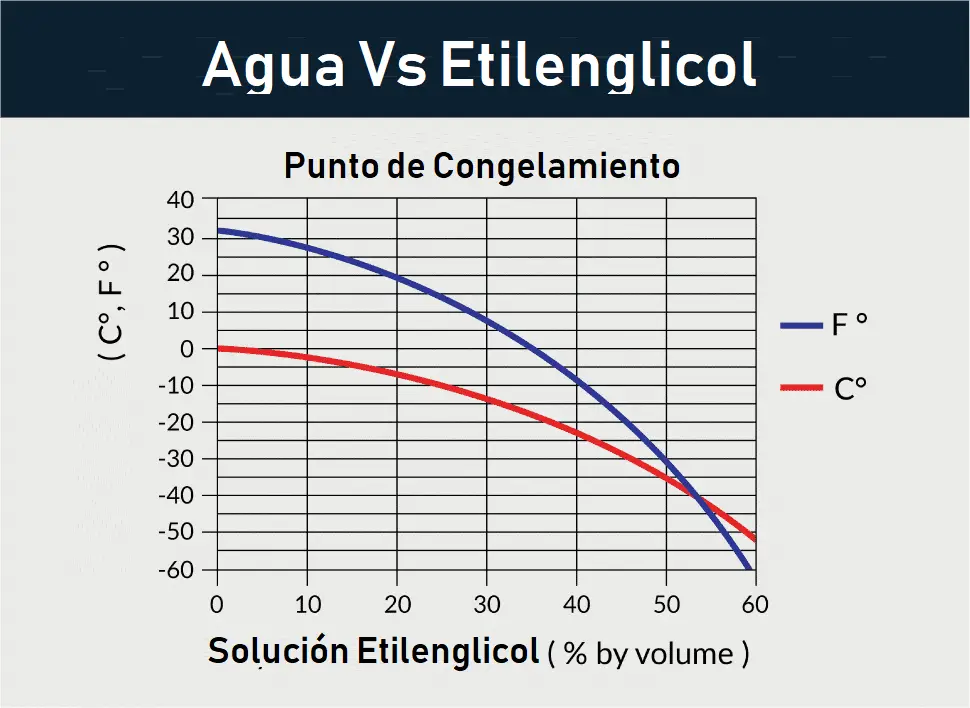
- Ethylene Glycol vs Freezing Point:
| Percentage | Freezing Temp. (°C) | Freezing Temp. (°F) |
|---|---|---|
| 5% | -1.1°C | 30.02°F |
| 10% | -2.2°C | 28.04°F |
| 20% | -6.7°C | 19.94°F |
| 30% | -12.8°C | 8.96°F |
| 40% | -20.6°C | -5.08°F |
| 50% | -33.3°C | -27.94°F |
Water Treatment for Chillers
Water treatment for chillers typically includes the following filtration steps:
- Filtration and Ultrafiltration
- Ion Exchange and Water Softening
- Chemical Additions
- Reverse Osmosis Systems
Detailed Explanation of Treatment Steps
- Filtration and Ultrafiltration:
Removes impurities such as organic matter and suspended solids from feed water. - Ion Exchange for Water Softening:
Systems remove ions (like magnesium, calcium, and iron) that increase water hardness, replacing them with non-hardness ions such as sodium. - Chemical Additions:
Chemicals like bicarbonates are added to prevent acidity and scale deposits. Pretreatment can reduce the quantity of chemicals needed, lowering costs. - Reverse Osmosis Systems:
Reverse osmosis eliminates almost all undesired dissolved solids, producing ultra-pure water.
Importance of Water Treatment for Chillers
Untreated water can contaminate heat transfer surfaces, reducing efficiency. Even a thin contamination layer significantly impacts performance.